An important step for the remote control of bioactive molecules?
Publication in Angewandte Chemie International Edition
A remote-controlled switching concept for selective switching on and off of proteins using ultrasound
The application possibilities of ultrasound are versatile and numerous. Due to its biocompatibility, it is commonly used in medical therapies and in vivo imaging. In addition, ultrasound is used to specifically cleave synthetic polymer chains to influence their properties and functions. Therefore, it would be an important milestone to use ultrasound to influence biological macromolecules, such as proteins, such that their optical or catalytic activities can easily be activated and deactivated.
A research team from Germany and the Netherlands has achieved a first breakthrough in this field. The scientists, among them Prof. Dr. Andreas Herrmann, Dr. Robert Göstl, and Dr. Arnold Boersma from the DWI - Leibniz Institute for Interactive Materials, have succeeded in switching the function of genetically engineered proteins on and off using ultrasound. For this, they added long and highly charged domains to the native structure of the proteins, rendering them sensitive to the shear forces induced by ultrasound.
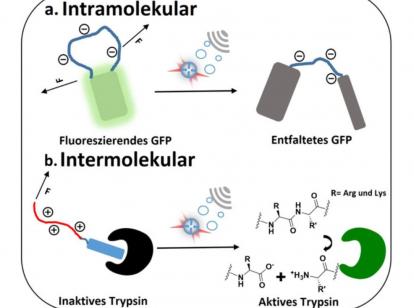
Exemplarily using GFP (green fluorescent protein), a frequently used marker protein, the researchers were able to show that the inserted domain is destabilized by the mechanical force induced by ultrasound. This caused the protein to lose its green fluorescence. However, the secondary structure of the protein remained intact. By specifically inserting this domain, the scientists were able to provide the otherwise ultrasound-stable protein with an ultrasound-sensitive "off switch".
In addition, the research team used the example of trypsin, a protease of the digestive system, to prove that the activity of proteins can not only be deactivated but also activated by ultrasound exposure. Therefore, they fused a charged long domain with a peptide that inhibits the activity of the enzyme. By applying ultrasound, the protein-protein interaction between enzyme and inhibitor was successfully interrupted releasing the active enzyme.
Thus, the researchers have established the first molecular concept for activating and deactivating the optical and catalytic activities of genetically engineered proteins by employing ultrasound and thus shear force in solution as a trigger. The use of external stimuli to control protein function is becoming increasingly relevant, as the growing field of optogenetics and its implications for the neurosciences and other areas show.
The research team also believes that these findings have laid a first important foundation for the remote control of protein activities in vivo, for example in the human body, and have thus created new potential for the development of innovative, non-invasive, biocompatible medical therapies and in vivo imaging techniques.