Mimicking cell membranes for a better understanding of biological processes and functions
Dr. Nina Kostina is a researcher at DWI - Leibniz Institute for Interactive Materials in the group of Dr. César Rodriguez-Emmenegger. Together with graduate students Dominik Söder, Anna Wagner, and Anton Joseph as well as Dr. Tamás Haraszti, she is working on the development of fully synthetic cells, so-called ʺprotocellsʺ. The researchers' aim is to mimic and study processes that take place in real biological cells.These minimalistic cells serve as a model to analyze and unravel complex biological processes - all the way to elucidating the origin of life.
The goals of this research are to design and fabricate synthetic building blocks that can self-assemble into vesicles, membrane-bound bubbles that mimic essential properties of cell membranes. The main focus is on the interaction with living matter and the question of how, for example, division and communication can be programmed in these synthetic cells. The team has recently made great progress in this regard and their work has been published in renowned journals such as Angewandte Chemie, PNAS, Nano Letters and Soft Matter (selected for the themed issue ʺRemodelling of Biomembranesʺ and featured on the cover).
The versatility and tasks of the cell membrane
Cell membranes have numerous functions and tasks, partly appearing contradictory at first glance. For example, they serve as a physical barrier to protect against external influences. On the other hand, they absorb stimuli from the environment and thus transmit targeted signals into the cell interior. In addition, they supply the cell with nutrients. Finally, they impart mechanical properties that enable the cell's flexibility and mobility.
The composition and structure of the cell membrane must therefore be versatile and complex, so that some questions quickly arise: What is a cell membrane actually made of? And how does it manage to fulfill such a wide range of functions? " The cell membrane is a very complex organelle, in simple terms an "organ of the cell", and consists of almost 80% lipids, the remaining components are proteins among others. The outmost interface of the cell is the glycocalyx. It consists of complex organizations of sugar-containing building blocks, such as glycolipids and glycoproteins. Not surprisingly, it is the first component to interact with the environment enabling very important cellular tasks, such as communication, nutrient delivery, signal transduction and interactions with the extracellular matrix." explains Nina Kostina. ʺHowever, due to the complex structure, it is almost impossible to look at and study the individual functions separatelyʺ, she says. "To achieve this, we need to develop a new model system that emulates the properties of cell membranes and allows the incorporation of biological building blocks," the chemist explains.
Dendrimersomes: Synthetic vesicles with special properties
Due to the high degree of complexity of biomembranes, many factors have to be considered when creating an artificial membrane model. The incorporation of biological building blocks requires that the thickness of the membrane is identical to the one of a natural cell membrane, i.e. always between 4 and 6 nanometers. These biological building blocks should be able to move freely across the membrane. This is referred to as lateral mobility. The membrane must be extremely flexible yet stable. However, designing such a thin construct to be stable without losing flexibility has been a mammoth task for researchers in recent years.
To meet this challenge, the DWI research team is collaborating with the group of Prof. Virgil Percec (University of Pennsylvania), one of the pioneers in the field of "Janus dendrimers" (JD). Analogous to the ancient Roman god Janus, these molecules have two ʺfacesʺ - so-called dendrons - attached to the opposite side of the "branched core." Thus, two opposite functionalities: water-loving (hydrophilic) and water-repellent (hydrophobic) are linked in one and the same molecule. In aqueous solutions, the JD assemble independently to form so-called vesicles. Experts refer to this process as "self-assembly." In the case of JD, the resulting vesicles are also called dendrimersomes. The advantage of this system is the possibility to “program” the properties of the dendrimersome into the structure of JD during the synthesis.
The researchers have already succeeded in creating these synthetic vesicles with promising properties such as a membrane thickness of 4 to 6 nanometers, high flexibility, lateral mobility and stability. The combination of these properties makes dendrimersomes cell membrane models that go beyond commonly used cell membrane mimics, such as liposomes and polymersomes.
How shape transformations can be mimicked on cell membranes
One goal pursued by the research team is to induce shape changes in the membrane model that naturally occur to cells in various situations. The vital functions of cell membranes require the ability to change their shape to perform complex tasks such as movement, division, and the uptake of substances by invagination of the membrane. All of these changes in cell shape are driven by very complex processes, such as changes in lipid composition, the clustering of curvature-stabilizing proteins and the reversible insertion of protein domains, which act like wedges in the membrane. But could much simpler mechanisms support membrane shape transformations?
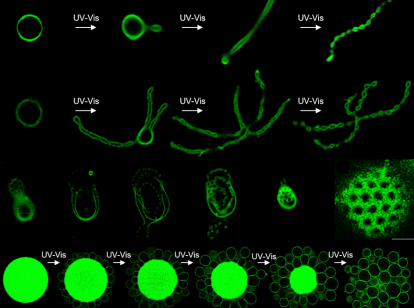
To answer this question, the research team went one step further in the development of dendrimersomes: they generated two JD variants. One variant was chemically designed in such a way that the large part of the hydrophilic branch is cleaved off using ultraviolet (UV) or visible (vis) light, so that the JD is no longer cylindrical but wedge-shaped in the membrane. The other variant is stable under the same light influences and retains its cylindrical shape. After the self-assembly of these two variants into vesicles, the scientists were able to influence their shape by irradiating them with UV‑vis light. The cleavage of a part of the molecules in the vesicles changes their shape leading to a much smaller hydrophilic area on the membrane. Thus, the total area of the membrane shrinks and reduces the volume of the vesicle. Furthermore, the high lateral mobility of the cylindrical JD allowed local concentration in the membrane, forcing an asymmetry that also induces curvature. Various shape transformations that are ubiquitous in cells and their organelles, such as budding, division, and pore formation, could thus be observed directly under the microscope for dendrimersomes as well. Some vesicles form a string of pearls, while others resemble a flower drawn by a child's hand.
One of the most important observations made by the scientists in this work: After exposure to light, a small protrusion forms from the dendrimersome membrane, which grows into a long tube and eventually turns into a bead chain-like vesicle. But what is special about this? This very process is reminiscent of processes that can be observed in naturally occurring cellular apoptosis, which is understood as "programmed cell death". When cells decide to commit apoptosis, that is, to initiate self-destruction, they undergo the same shape changes as observed in dendrimersomes. One of the mechanisms driving shape transformations during apoptosis involves the enzymatic cleavage of cylindrical lipids (sphingomyelin) into wedge-shaped lipids (ceramide). Thus, both processes are based on a similar principle and the results provide a valuable basis for the study of real cellular membrane processes.
How do the molecular patterns of glycocalyx affect its biological activity?
In another research project, the researchers addressed the question of how the molecular patterns present in the glycocalyx affect its biological activity. The variety and high complexity of functions of the cellular glycocalyx arise from the combination of the chemical diversity of sugar moieties and their spatial three-dimensional arrangement that control the affinity in sugar-protein interaction. The sugar moieties on the cell membrane are organized in unique molecular patterns that, among other things, define the cell as endogenous, but are also exploited by pathogenic bacteria and viruses to attack the cells. Therefore, it is not surprising that much attention has been paid to the glycocalyx. Scientists are trying to clarify how function results from the spatial arrangement of sugar groups on the cell membrane. However, despite decades of work, the structure of the glycocalyx in cells is difficult to elucidate due to the limitations of imaging techniques, even with the most advanced ultra-high resolution microscopes.
Recently, the group around Dr. César Rodriguez-Emmenegger, again together with the group of Prof. Virgil Percec, has developed rather special glycodendrimersomes. These are dendrimersomes that carry sugar building blocks outside of the membrane. For this work, the simplest sugar group – mannose – was selected due to its high biological relevance and presence in the cellular glycocalyx. After mannose-decorated JD self-assembled into vesicles, the research team performed a meticulous analysis of their membranes and found out that the sugar moieties arrange themselves in a very peculiar mosaic pattern on the membrane. During the studies, they revealed that these special patterns of the sugar dramatically increase the binding affinity to concanavalin A, which is a protein that specifically binds mannose and is already used in immunological research, among other applications.
Understanding the formation of periodic sugar nanoarrays, i.e. the mosaic patterns of the glycocalyx, can contribute to a better understanding of the processes involved in cell communication, signal transduction, and vesicle transport within cells. It provides a powerful example of how functions can be affected by structure. Of particular interest is how different molecular arrangements determine biological recognition. This knowledge may be of great importance in biomedicine for the development of therapeutics that distinguish between host cells and pathogens such as viruses or bacteria.