Ultrasound to make therapeutic nucleic acids more effective
Aman Ishaqat, scientist at DWI – Leibniz Institute for Interactive Materials conducts research in Prof. Andreas Herrmann's group in the field of therapeutic nucleic acids. These have enormous potential for treating a variety of human diseases that, for example, damage nerves or lead to increased muscle loss. With her research, Aman wants to expand the application possibilities of nucleic acids, as well as making their clinical-therapeutic use more efficient.
Since the beginning of the prevailing pandemic, caused by the coronavirus SARS-CoV-2, everyone pays increased attention when RNA is mentioned. For example, the membrane-enveloped virus uses its own RNA to replicate itself at the target site like the lung tissue of the human body and cause harm. RNA is also used in vaccine research, where it serves as a blueprint for viral components, or more precisely proteins. These activate the immune system and prepare the body for a real infection. Furthermore, nucleic acids have also been utilized in the clinical-therapeutic field in recent years. Over time more and more possibilities for new therapeutic approaches emerge.
What makes therapeutic nucleic acids so special?
Therapeutic nucleic acids are basically genetic information or blueprints that can consist of both DNA and RNA, thus deoxy- or ribonucleic acid, and offer new possibilities in the treatment of a variety of human diseases. Some of these diseases have been "undruggable" with conventional drugs or proteins, for example due to a lack of ability to target the disease origin or sites of action. Other advantages of RNA therapeutics include their comparatively rapid production, as well as the ability to tailor their sequences quickly and specifically. However, despite their potential, there are many challenges that need to be addressed to achieve greater success of therapeutic nucleic acids in clinical applications. Aman's research addresses some of these challenges. For instance, the nucleic acids shall be delivered in a controlled manner to the desired location in the human body at the right time and dose. In doing so, the nucleic acids can be used to either stimulate or inhibit specific processes within the cell. The potential applications of new therapeutic approaches range from the field of sonopharmacology, where the focus is on drug activation, to the field of sonogenetics, where protein and gene functions are targeted.
The way into our body
To ensure that nucleic acids can perform the desired functions, they must be delivered to the target site in our body in a gentle manner. RNA in particular, is considered as an unstable molecule and can be easily degraded by ubiquitous enzymes. To circumvent these problems, the researcher utilizes structural modifications and so-called transport systems. Among others, tiny structures are attached to the respective ends of the mRNA (messenger RNA) molecules to protect them from unwanted degradation or decay. In another approach, DNA nanostructures synthesized specifically for this purpose are combined with polymers. Thus, on the one hand they help the drug to reach its intended site of action unharmed and on the other hand interface with surfaces and membranes at the target site.
Smart drug delivery systems
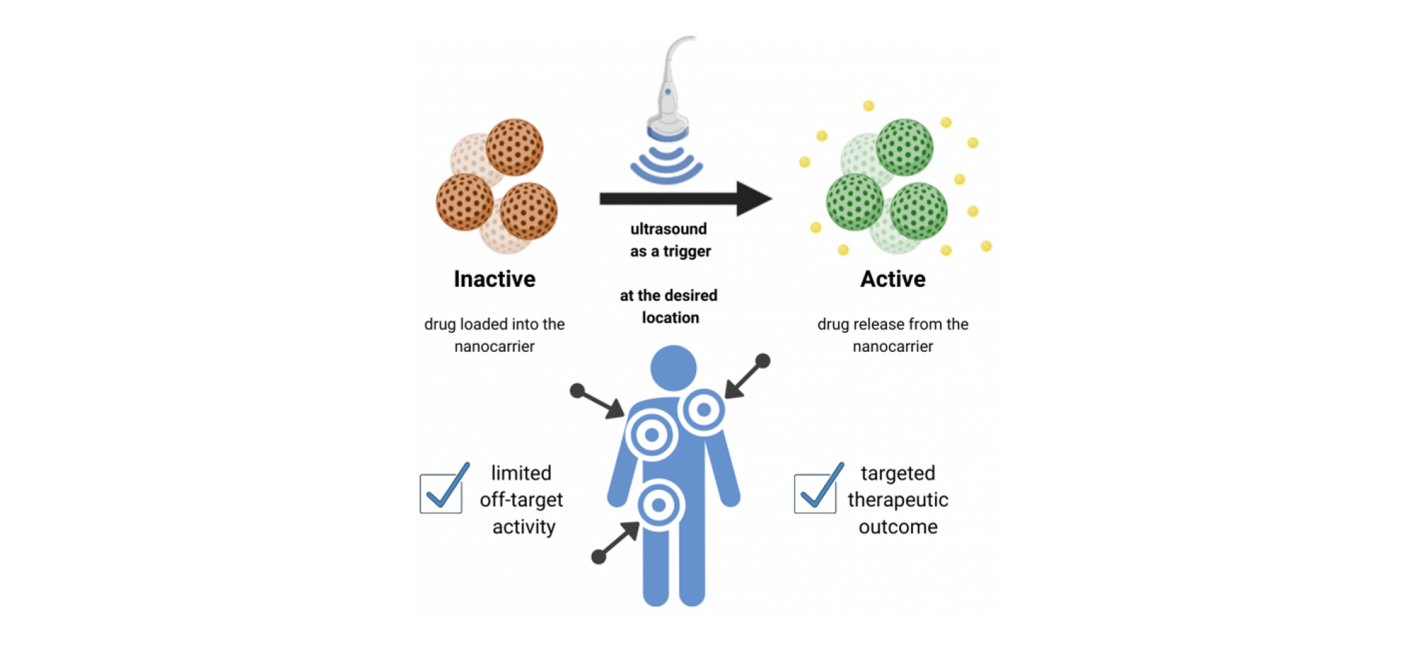
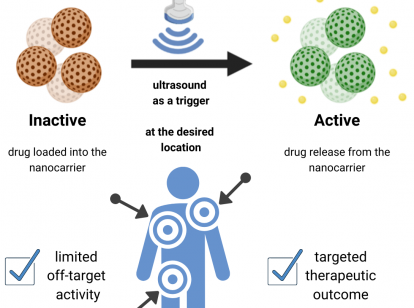
Once the RNA molecules have arrived at the desired location, these modifications are then to be targeted for cleavage using low-energy ultrasound, which is also used in numerous other clinical applications. This functional principle is called smart drug delivery system in its entirety and additionally makes use of DNA-based nanotechnology. The cleavage from the transport molecule and the resulting release of the therapeutic nucleotides thus offers a non-invasive approach to the treatment of some human diseases. The transport system is designed to bind therapeutics while inactivating and protecting them until release is triggered by ultrasound from outside the body.
Aman's ambition is to develop systems to deliver therapeutic nucleic acids to specific tissues in the human body, where they can exert different effects. Among other things, she is using a DNA construct that stimulates the immune system and could be used in combination with antigens for cancer immunotherapy. Another focus of her work is the delivery of special siRNA (small interfering RNA), which can be used for gene knockdown. This means that a specific target gene can be switched off and its expression, thus the subsequent formation of the corresponding proteins, can be prevented in the cell.
Maximizing drug efficiency and minimizing side effects
The number of approved drugs that belong to the class of therapeutic nucleic acids and are suitable for human use is very limited. The ongoing research explores systems that can be used for efficient delivery of nucleic acids and holds much potential for future medical therapies to treat many diseases, including cancer, infections, as well as genetic disorders. In the field of cancer immunotherapy, stimulating the immune system to attack cancer cells is one of the most recently developed approaches for treatment of cancer. Furthermore, the use of siRNA could enable the selective switching off of genes to treat diseases in which genetic defects lead to the overproduction of a certain protein.
Overall, stimulus-responsive systems have many advantages over conventional drug delivery methods, especially when the trigger is minimally invasive and has high penetration depth in tissue. By using ultrasound in the case of the smart drug delivery system, the majority of the drug will be localized at the specific tissue where it should perform its activity. This means maximizing the efficiency of the drug and minimizing the potential side effects. Thus, the overall therapeutic outcome and the patient's quality of life could be improved in the future.