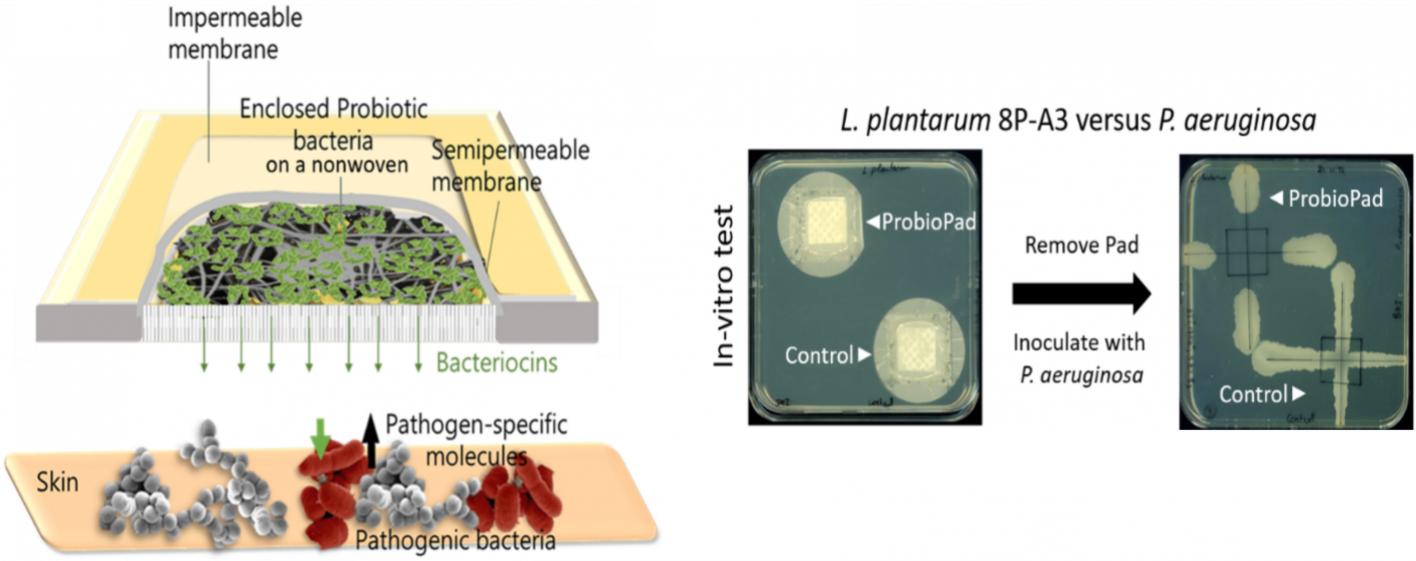
The project within the framework of the NRW patent validation is based on the invention for the treatment of skin diseases using probiotic bacteria and their application in the form of a skin plaster. The invention addresses a medical need and aims to cure common skin diseases without serious side effects and without or with reduced use of antibiotics in order to avoid the development of antibiotic resistance.

The funding enables the target-oriented development and application-oriented validation of the invention in compliance with medical guidelines and GMP conformity. The invention is to be classified in the innovation field "Innovative Medicine, Health and Life Science" and the focus "Materials for Biomedicine" and aims to create a biologized therapeutic system for medicine. The validation of the invention, which is made possible by the EFRE/NRW funding, serves to gain information for the preparation of clinical trials as a prerequisite for further development towards market readiness. Towards the end of phase 1, cooperation partners are involved and potential companies are identified for commercialization. If successful, the further development will lead to an improved quality of life for the people affected and will therefore also be of great benefit to society.
EFRE-20800067
Implementation period 15.11.2023 – 14.05.2025