"Hot Paper" in Applied Chemistry In the cage to the place of action
How do you transport a highly effective active ingredient to the exact place where it is supposed to act in the body? Chemists at the DWI - Leibniz Institute for Interactive Materials, together with colleagues from Heinrich Heine University Düsseldorf (HHU), present an approach to solving this problem in the journal Angewandte Chemie. The idea: The use of a molecular cage that opens upon ultrasound irradiation.
In supramolecular chemistry, molecules are assembled into larger, superordinate structures. With a suitable choice of subcomponents, such systems assemble themselves from their individual building blocks; this is referred to as self-assembly.
Certain supramolecular compounds are well suited as so-called host-guest systems. In such cases, a host structure surrounds a guest molecule, which can shield, protect and transport it from the environment. This is a specialty of Dr. Bernd M. Schmidt and his research group at the Institute of Organic Chemistry and Macromolecular Chemistry at HHU in Düsseldorf.
The Düsseldorf chemists, together with colleagues from the DWI - Leibniz Institute for Interactive Materials, were looking for a system that might later even be able to transport drug molecules through the human body and, above all, release the cargo at a desired location.
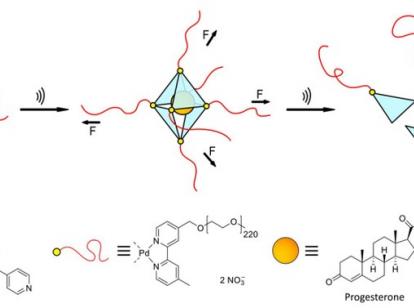
The solutions can be discrete "Pd6(TPT)4 cages": octahedron-shaped structures consisting of four triangular panels, at each of whose six corners sits a large organic molecule, more precisely a palladium atom, over which a long polymer chain is bonded. These palladium atoms form so-called coordinative metal bonds to the panels whose corners they abut.
If the individual building blocks are added to an aqueous solution in the correct ratio, the cages form by themselves. If smaller, hydrophobic molecules are subsequently added, they migrate into the cages in precisely known numbers. The researchers have demonstrated this with the active ingredient molecules ibuprofen (painkiller) and progesterone (luteal hormone).
"The special feature of our system is the built-in predetermined breaking points," said Dr. Schmidt, last author of the study. "The palladium atoms bind all the building blocks comparatively weakly. If you manage to pull them out of the bond, the whole octahedron breaks apart," he explains.
In this case, "disintegration" takes place in Aachen - using powerful ultrasound sources such as those used in medicine to disintegrate kidney stones. The ultrasound generates cavitation bubbles in the water. These burst, thereby exerting very large mechanical shear forces on the long chains. The forces are so strong that they literally tear the palladium atoms out of their corners, thus disintegrating the octahedral cage. The small active ingredient molecules are whirled around in the process, but not damaged.
"Local ultrasound irradiation of the tissue to be treated could later be used to ensure that the active ingredient transported in the cage is released exactly where it is needed for therapy," explains Dr. Robert Göstl, an expert in the field of polymer mechanochemistry from Aachen. The active ingredient molecules used in the study serve only as a test. In principle, it is possible for very many different hydrophobic molecules to be packed into the cage. And - unlike other host-guest systems described - it is not necessary to chemically adapt the drug molecules to get them into the cage. "For the treatment of cancer, for example, it would be conceivable to load cytostatic drugs. By releasing them directly at a solid tumor, perhaps chemotherapy could be carried out with significantly less active ingredient and thus with fewer side effects," Bernd M. Schmidt hopes.
What's more, the defined loading quantity also makes it possible to measure exactly how much active ingredient is released at the point of use. "The dose administered could thus even be calculated precisely," says Bernd M. Schmidt.
The study is a so-called "proof of concept," which means that the feasibility of the approach was demonstrated. This also convinced the reviewers and publishers of the journal "Angewandte Chemie", who considered the publication to be particularly significant. It has been classified as a "hot paper" and made the cover story of the next journal issue.
"The next step is experiments in which we want to check how human cells react to our cages. This is necessary because before they can be used for medical purposes, it must be ensured that they do not have a toxic effect on the cells," explains Bernd M. Schmidt.